Table of Contents
IMPORTANCE OF DNA PROFILING AND GENETIC INFORMATION FOR STATE AGENCIES
DNA PROFILING
INTRODUCTION
DNA profiling is the process where a specific DNA pattern, called a profile, is obtained from a person or sample of bodily tissue .
Even though we are all unique, most of our DNA is actually identical to other people’s DNA. However, specific regions vary highly between people. These regions are called polymorphic. Differences in these variable regions between people are known as polymorphisms. Each of us inherits a unique combination of polymorphisms from our parents. DNA polymorphisms can be analysed to give a DNA profile.Human DNA profiles can be used to identify the origin of a DNA sample at a crime scene or test for parentage.
DNA profiling is a forensic technique in criminal investigations, comparing criminal suspects’ profiles to DNA evidence so as to assess the likelihood of their involvement in the crime. It is also used in parentage testing, to establish immigration eligibility, and in genealogical and medical research. DNA profiling has also been used in the study of animal and plant populations in the fields of zoology, botany, and agriculture.
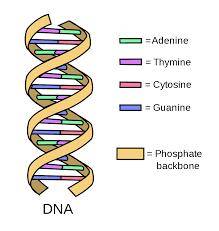
Deoxyribonucleic acid is a molecule composed of two polynucleotide chains that coil around each other to form a double helix carrying genetic instructions for the development, functioning, growth and reproduction of all known organisms and many viruses. DNA and ribonucleic acid (RNA) are nucleic acids. Alongside proteins, lipids and complex carbohydrates (polysaccharides), nucleic acids are one of the four major types of macromolecules that are essential for all known forms of life.
The structure of the DNA double helix. The atoms in the structure are colour-coded by element and the detailed structures of two base pairs are shown in the bottom right.
The two DNA strands are known as polynucleotides as they are composed of simpler monomeric units called nucleotides. Each nucleotide is composed of one of four nitrogen-containing nucleobases (cytosine [C], guanine [G], adenine [A] or thymine [T]), a sugar called deoxyribose, and a phosphate group. The nucleotides are joined to one another in a chain by covalent bonds (known as the phospho-diester linkage) between the sugar of one nucleotide and the phosphate of the next, resulting in an alternating sugar-phosphate backbone. The nitrogenous bases of the two separate polynucleotide strands are bound together, according to base pairing rules (A with T and C with G), with hydrogen bonds to make double-stranded DNA. The complementary nitrogenous bases are divided into two groups, pyrimidines and purines. In DNA, the pyrimidines are thymine and cytosine; the purines are adenine and guanine.
Both strands of double-stranded DNA store the same biological information. This information is replicated as and when the two strands separate. A large part of DNA (more than 98% for humans) is non-coding, meaning that these sections do not serve as patterns for protein sequences. The two strands of DNA run in opposite directions to each other and are thus antiparallel. Attached to each sugar is one of four types of nucleobases (or bases). It is the sequence of these four nucleobases along the backbone that encodes genetic information. RNA strands are created using DNA strands as a template in a process called transcription, where DNA bases are exchanged for their corresponding bases except in the case of thymine (T), for which RNA substitutes uracil (U).[4] Under the genetic code, these RNA strands specify the sequence of amino acids within proteins in a process called translation.
Within eukaryotic cells, DNA is organized into long structures called chromosomes. Before typical cell division, these chromosomes are duplicated in the process of DNA replication, providing a complete set of chromosomes for each daughter cell. Eukaryotic organisms (animals, plants, fungi and protists) store most of their DNA inside the cell nucleus as nuclear DNA, and some in the mitochondria as mitochondrial DNA or in chloroplasts as chloroplast DNA.[5] In contrast, prokaryotes (bacteria and archaea) store their DNA only in the cytoplasm, in circular chromosomes. Within eukaryotic chromosomes, chromatin proteins, such as histones, compact and organize DNA. These compacting structures guide the interactions between DNA and other proteins, helping control which parts of the DNA are transcribed.
BACKGROUND
Starting in the 1980s scientific advances allowed the use of DNA as a material for the identification of an individual. The first patent covering the direct use of DNA variation for forensics was filed by Jeffrey Glassberg in 1983, based upon work he had done while at Rockefeller University in 1981. In the United Kingdom, Geneticist Sir Alec Jeffreys independently developed a DNA profiling process beginning in late 1984 while working in the Department of Genetics at the University of Leicester.
The process, developed by Jeffreys in conjunction with Peter Gill and Dave Werrett of the Forensic Science Service (FSS), was first used forensically in the solving of the murder of two teenage girls who had been raped and murdered in Narborough, Leicestershire in 1983 and 1986. In the murder inquiry, led by Detective David Baker, the DNA contained within blood samples obtained voluntarily from around 5,000 local men who willingly assisted Leicestershire Constabulary with the investigation, resulted in the exoneration of a man who had confessed to one of the crimes, and the subsequent conviction of Colin Pitchfork. Pitchfork, a local bakery employee, had coerced his coworker Ian Kelly to stand in for him when providing a blood sample; Kelly then used a forged passport to impersonate Pitchfork. Another coworker reported the deception to the police. Pitchfork was arrested, and his blood was sent to Jeffrey’s lab for processing and profile development. Pitchfork’s profile matched that of DNA left by the murderer which confirmed Pitchfork’s presence at both crime scenes; he pleaded guilty to both murders.
Although 99.9% of human DNA sequences are the same in every person, enough of the DNA is different that it is possible to distinguish one individual from another, unless they are monozygotic (identical) twins.[12] DNA profiling uses repetitive sequences that are highly variable,[12] called variable number tandem repeats (VNTRs), in particular short tandem repeats (STRs), also known as microsatellites, and minisatellites. VNTR loci are similar between closely related individuals, but are so variable that unrelated individuals are unlikely to have the same VNTRs.
DNA PROFILING IS USED TO:
Identify the probable origin of a body fluid sample associated with a crime or crime scene reveal family relationships identify disaster victims, for example, ESR scientists travelled to Thailand to help identify victims of the 2004 Boxing Day tsunami.
What are short tandem repeats?
One of the current techniques for DNA profiling uses polymorphisms called short tandem repeats. Short tandem repeats (or STRs) are regions of non-coding DNA that contain repeats of the same nucleotide sequence. For example, GATAGATAGATAGATAGATAGATA is an STR where the nucleotide sequence GATA is repeated six times. STRs are found at different places or genetic loci in a person’s DNA.
What is a DNA profile?
One way to produce a DNA profile, is for scientists to examine STRs at 10 or more genetic loci. These genetic loci are usually on different chromosomes. A DNA profile can tell the scientist if the DNA is from a man or woman, and if the sample being tested belongs to a particular person.
How do you create a DNA profile using STR?
1. Get a sample of DNA
DNA is found in most cells of the body, including white blood cells, semen, hair roots and body tissue. Traces of DNA can also be detected in body fluids, such as saliva and perspiration because they also contain epithelial cells. Forensic scientists and Police officers collect samples of DNA from crime scenes. DNA can also be collected directly from a person using a mouth swab (which collects inner cheek cells). Find out more in the article Crime scene evidence.
2. Extract the DNA
DNA is contained within the nucleus of cells. Chemicals are added to break open the cells, extract the DNA and isolate it from other cell components.
3. Copy the DNA
Often only small amounts of DNA are available for forensic analysis so the STRs at each genetic locus are copied many times using the polymerase chain reaction (PCR) to get enough DNA to make a profile. Find out more in the article What is PCR? Specific primers are used during PCR that attach a fluorescent tag to the copied STRs.
4. Determine the size of the STRs
The size of the STRs at each genetic locus is determined using a genetic analyser. The genetic analyser separates the copied DNA by gel electrophoresis and can detect the fluorescent dye on each STR. This is the same piece of equipment used in the lab for DNA sequencing.
5. Is there a match?
The number of times a nucleotide sequence is repeated in each STR can be calculated from the size of the STRs. A forensic scientist can use this information to determine if a body fluid sample comes from a particular person.
GENETIC INFORMATION
INTRODUCTION
two DNA profiles from different samples are the same, the chance that the samples came from different people is low. This provides strong evidence that the samples have a common source. To produce a DNA profile, scientists examine STRs at ten, or more, genetic loci. These genetic loci are usually on different chromosomes.
Genetic information of an organism is stored in DNA molecules. How can one kind of molecule contain all the instructions for making complicated living beings like ourselves? What component or feature of DNA can contain this information? It has to come from the nitrogen bases, because, as you already know, the backbone of all DNA molecules is the same. But there are only four bases found in DNA: G, A, C, and T. The sequence of these four bases can provide all the instructions needed to build any living organism. It might be hard to imagine that 4 different “letters” can communicate so much information. But think about the English language, which can represent a huge amount of information using just 26 letters. Even more profound is the binary code used to write computer programs. This code contains only ones and zeros, and think of all the things your computer can do. The DNA alphabet can encode very complex instructions using just four letters, though the messages end up being really long. For example, the E. coli bacterium carries its genetic instructions in a DNA molecule that contains more than five million nucleotides. The human genome (all the DNA of an organism) consists of around three billion nucleotides divided up between 23 paired DNA molecules, or chromosomes.
The information stored in the order of bases is organized into genes: each gene contains information for making a functional product. The genetic information is first copied to another nucleic acid polymer, RNA (ribonucleic acid), preserving the order of the nucleotide bases. Genes that contain instructions for making proteins are converted to messenger RNA (mRNA). Some specialized genes contain instructions for making functional RNA molecules that don’t make proteins. These RNA molecules function by affecting cellular processes directly; for example some of these RNA molecules regulate the expression of mRNA. Other genes produce RNA molecules that are required for protein synthesis, transfer RNA (tRNA), and ribosomal RNA (rRNA).
In order for DNA to function effectively at storing information, two key processes are required. First, information stored in the DNA molecule must be copied, with minimal errors, every time a cell divides. This ensures that both daughter cells inherit the complete set of genetic information from the parent cell. Second, the information stored in the DNA molecule must be translated, or expressed. In order for the stored information to be useful, cells must be able to access the instructions for making specific proteins, so the correct proteins are made in the right place at the right time.
Structure of DNA double helix. Sugar-phosphate backbone is shown in yellow, specific base pairings via hydrogen bonds (red lines) are colored in green and purple (A-T pair) and red and blue (C-G). Both copying and reading the information stored in DNA relies on base pairing between two nucleic acid polymer strands. Recall that DNA structure is a double helix.
The sugar deoxyribose with the phosphate group forms the scaffold or backbone of the molecule (highlighted in yellow in Figure 1). Bases point inward. Complementary bases form hydrogen bonds with each other within the double helix. See how the bigger bases (purines) pair with the smaller ones (pyrimidines). This keeps the width of the double helix constant. More specifically, A pairs with T and C pairs with G. As we discuss the function of DNA in subsequent sections, keep in mind that there is a chemical reason for specific pairing of bases. To illustrate the connection between information in DNA and an observable characteristic of an organism, let’s consider a gene that provides the instructions for building the hormone insulin. Insulin is responsible for regulating blood sugar levels. The insulin gene contains instructions for assembling the protein insulin from individual amino acids. Changing the sequence of nucleotides in the DNA molecule can change the amino acids in the final protein, leading to protein malfunction. If insulin does not function correctly, it might be unable to bind to another protein (insulin receptor). On the organismal level of organization, this molecular event (change of DNA sequence) can lead to a disease state—in this case, diabetes.
METHODS
The method used for analyzing the professional and scientific views on the social, ethical, and legal issues that impact on genetic information and testing in insurance and employment in Europe was primarily the review of the technical, social, economical, and ethical aspects of advances in genetics and the concerns of parties who are involved, that is, the insurers, the employers, and the public. The existing guidelines and legislation on this topic were also reported. Then, the method was to examine the issues debated by these parties in Europe, as well as the results of discussions held during an international workshop. This workshop was organized by the European Society of Human Genetics Public and Professional Policy Committee in Manchester, United Kingdom, February 25–27, 2000.
The purpose of the workshop was to identify, from a professional viewpoint, the most important/pressing/burning ethical issues raised by genetic information and testing in insurance and employment in Europe. The formal workshop presentations covered the following themes: the fundamentals of genetics, of insurance, family histories, actuarial relevance and genetic testing and employment issues. Small multidisciplinary groups were convened to take these discussions further, in particular to consider the specific issues involved in employment, life insurance, private medical insurance, long-term care and critical illness insurance, and total permanent disability and income replacement insurance. Their initial task was to explore the insurance needs and rights in the countries represented and to consider the extent to which these needs were currently being met. Following the small group sessions, conclusions were fed back to the whole group where there were opportunities for further discussion.
group of 47 experts from 14 European countries was invited. These experts were representatives of the seven following sectors:
1)Medical Genetics
2)Human Genetics Societies
3)Ethical, Legal and Social Issues
4)Support Groups
5)Biotechnology/Pharmaceutics
6)Insurance/Employment
7)European Union Institutions
A first background document was discussed during the workshop. A second document, including discussions of the workshop, was sent for comments to representatives of the human genetic societies and European experts in the fields of insurance and genetics, as well as to all ESHG members. This document was also put on the ESHG website (www.eshg.org) for public consultation and discussion. The final document was approved by the ESHG board.
Concerns of medical geneticists
Complexity of genetic tests
Genetic tests are available for two forms of genetic diseases: monogenic and multifactorial diseases. Monogenic disorders are rare but highly penetrant; the genetic test will indicate whether a person has or will get the disease. Presymptomatic testing identifies healthy individuals who may have inherited a gene for a late-onset disease and if so will develop the disease if they live long enough. Multifactorial diseases are frequent and most likely triggered by specific combinations of functional DNA polymorphisms interacting with the environment in ways that are subject to behavioral changes. Susceptibility testing identifies healthy individuals who may have inherited a gene that puts them at increased risk of developing a multifactorial disease, although these individuals may never develop the disease in question. In these situations, the most that the genetic test can do is to show a propensity to a disease.1,2
Genetic testing classifies people into those who have the mutant gene and those who do not have it. Now, a mutant gene is not a disease. Genetic disorders show different degrees of severity and diverge with respect to the age of onset. Some genetic disorders affect people with near-certainty but others not. Predictions are therefore complicated by these phenomena., our ability to identify individuals at risk for genetic diseases often exceeds our ability to prevent or treat the diseases.3,4 This has been described as the ‘therapeutic gap’ and as a reason for tension between policy-makers and health professionals.5 The use of computerized medical data banks within large companies could exacerbate this problem, genetic information becoming not only a medical fact but also a disease.6,7
We are forced to note that genetic tests present some limits, including the possibility of uninformative results, the inability to predict the exact age of onset or the severity of symptoms and, in the case of multifactorial diseases, the inability to predict if the individuals will develop the disease in question. In fact, genetic tests cannot account adequately for the external factors, which can be as important as inborn characteristics.3,6,8 Tests using genetic markers linked to a disease gene (as opposed to testing directly for disease-causing mutations) are not totally reliable since they provide only statistical probabilities based on the presumption that people have inherited genes with the identified markers. In other respects, a clear distinction must be drawn between genetic tests carried out in a research setting (aimed at establishing new genetic tests or developing quality control of tests) and those carried out in clinical practice. Research projects can be experimental and the results of the tests can be uncertain.
Calling ethical principles into question
Different arguments suggest that there is something special about genetics, and yet ethical principles in medical genetics are the same in medicine, even if this has been questioned. These principles are: respect for the autonomy of persons, beneficence, non-maleficence, and justice. At present, in regard to medical genetics, these principles are not applied with equal force around Europe. The principle of nonmaleficence which aims at avoiding and preventing harm to persons, is called into question if genetic information is used for discrimination or favoritism in insurance and employment. The principle of justice, which may consist in distributing benefits and burdens fairly and with equity, varies depending on whether healthcare is founded on the principle of social solidarity or on the basis of mutuality. Although the market for private health insurance in Europe is small and in some countries nonexistent, the possible use of genetic information in insurance and employment has gradually generated debate and increasingly causes concern. Furthermore, medical geneticists’ concerns extend beyond the traditional ethical guidelines in medicine. For instance, genetic testing for insurance and employment purposes could disturb family relations. Family cooperation is often necessary to detect genetic problems, but genetic information may affect an entire family rather than only one individual, and the choices of the present may affect future generations. Genetic information links the members not only of families but also of whole communities. Genetic disorders are often over-represented in ethnic groups and intensive genetic research on some populations could exaggerate the presence of problems. of the insurers
Genetic information through family history was already used by some insurance companies before anyone considered genetic testing, and individuals were covered or denied coverage or charged higher premiums according to family history. Nevertheless, the progress made in predicting diseases alters the information available with regard to the risk of disease. Genetic information contains more certainty than information traditionally gathered by insurers to investigate the existence of diseases running in the family.6,18 This may have important consequences for insurance industry.
Goal of insurers
The insurers’ goal is to maximize their profits. This is usually reached with an increased number of people under coverage. In this regard, developments in medical science have resulted in an increase of life insurance sales.19 In other respects, everyone carries some potentially abnormal genes and insurers will not wish to deny coverage to a significant segment of the population. However, the insurance industry would like to use genetic information as just part of the (predictive) information that they should be able to use less for deciding to accept a private, voluntary application, than for setting the premium level according to the individuals’ risk, and for avoiding the possibility of adverse selection.
Underwriting
Underwriting is the method used to classify people according to their risk. Insurers classify the risk by asking questions and through medical investigation. The questions sometimes cover the medical histories of family members. Depending on the case and the amount of coverage involved, medical questions might be followed by medical tests or complete medical examinations.20,21
In the underwriting process, the expectations of individuals in relation to longevity and health are quantified and expressed as statistical probabilities. Insurers can predict that the overall mortality rate of a specific group of people, classified in the same substandard risk category, will be higher than the mortality rate in the general population.7 Usually, underwriting leads to classification in three groups: standard, substandard, and uninsurable. Individuals in the first group have few problems getting insurance. Individuals in the second group must pay higher than average premiums, based on the risk they represent. Individuals in the third group are excluded because the cost of their coverage is unquantifiable or would exceed any reasonable premium.
Experience shows that the assessment of substandard risks due to genetic information is proved fair since the observed mortality is very close to what had been expected. Requesting genetic tests from insurance applicants could then constitute another source of information for insurers. This would permit to classify individuals more accurately in various categories of risk, or to assess risk premiums more accurately. Genetic testing would enhance equity by allowing a precise calculation of which people are really in the same situation and which are not.6,18 The concept of equity in insurance means that people who have similar health or similar life expectancies should pay equal premiums and those who have worse health or lower life expectancies should pay more.
To date, insurers do not require applicants to submit to genetic testing. In some countries, this is due to legal barriers which prohibit insurers from asking for genetic tests. This is also due to the lack of information on the predictive value of certain tests and on the costs of diseases.1,6,22 But that does not mean that insurers are not using genetic information. Insurers can currently make genetic inferences from routine and well-accepted questions on family history. Insurers can use genetic information available in medical files; the registered information in medical files is usually more accurate and complete than what is known by the insurance applicants.15 Since genetics is integrated in medical practice, insurers will have access more and more to genetic information. This will allow insurers, among other things, to know whether applicants have neglected to mention that they are carriers of genetic disorders or that these run in the family.
Adverse selection
Adverse selection occurs when people have undergone testing and conceal positive test results from insurers.16 If the insured person does not disclose information which the insurer needs to know, then this disrupts the equilibrium of the relationship and the possibility of adverse selection arises. Insurers require symmetry of information. If insurers are prohibited from having access to pertinent information at the time of underwriting or when the policy is renewed, the applicants could use genetic information to abuse the insurance system, taking advantage of private knowledge of the risks they are submitting for coverage.19 The consequences of a lack of symmetry in information between insurers and applicants or insured persons could force insurers to adjust premiums. In this way, in the Netherlands, after the Medical Examination Act has been in force (1998), insurers have taken measures to prevent the risk of adverse selection by implementing premium increases in advance, by prescribing a maximization of the pension pay-out or basing payments on a maximum salary, or by including an option to increase the premium in the policy.23 Dutch insurers have also introduced waiting times for existing illnesses when issuing the insurance. This means that if, within a term stipulated in the waiting time, the insured becomes disabled or dies as a result of an illness that he had when he took out the insurance, no payment will be made. This measure does not apply for life insurance. Sweden (1999) has the same policy. In the United Kingdom (2000), the Genetics and Insurance Committee stated that the reliability and relevance of the genetic test for Huntington’s Disease was sufficient for insurance companies to use the result when assessing applications for life insurance. But in October 2001 the UK government reached an agreement with the Association of British Insurers (ABI) to institute a 5-year moratorium on the use of genetic tests results up to a certain value.
Concerns of the employers
Concerns of employers and of insurers are similar. The main difference between life insurers and employers is that for employers, sickness represents a greater financial risk than death, while for health insurers, the opposite is usually the case.
Goal of employers
It is in employers’ interests to have a healthy workforce. Some employers provide facilities to encourage the staff to achieve a good health, like regular medical check-ups and sport. It has been argued that if it could be demonstrated that genetic screening would encourage more healthy lifestyles, it would be possible to envisage that employers would fund such screening for their staff.23,24
Employers are particularly interested in the health of the employees for jobs where there is a substantial investment in training or for very senior positions. Different sources of information can be used to assess whether an individual has a risk of either sickness or death: medical examination, medical history, family history, age, lifestyle. Genetic testing might confirm the risk of developing a genetic disease, for which some jobs could make the person unacceptable.23,25 What would also change is that some employees would move from 50 to 0% chance and they would have opportunities which are currently denied them.
For most jobs, employers do not insist on intensive health testing of prospective employees, because the extent of the employers’ investment in new employees is not great enough to warrant such expense.23 The prospective employees are simply asked to make a declaration about their state of health.
Constraints imposed on employers
The costs of any health investigation by employers are significant: if employers investigate every prospective employee, they will have to pay the investigation costs for all of them, but in only a few will the investigation show anything at all. The decision for employers, where there is a known health risk, is whether the value that employees will give to the firm justifies the risk.21,23
Many employers provide a range of health insurance coverage for their employees: sick pay, permanent health insurance, spouse’s pension, retirement pensions, health-care benefits. Most employees are covered without having to provide any information about their health. But in recent years there has been some trend towards flexible remuneration packages, under which employees get some measure of choice as to which employees benefits they take. Where employees have a choice, some measure of individual underwriting is required.21,23
Although the use of genetic information might conceivably be of some benefit for employers, it runs counter to the fundamental rights of workers to nondiscrimination for health reasons and those relating to protection of privacy. For instance in France, such rights which have been reinforced by the laws on bioethics in 1994, are proclaimed in several articles in the labor and penal codes. In those countries that do not have specific regulations prohibiting or limiting employer access to, and use of, genetic information, existing antidiscrimination and privacy legislation may provide individuals with some protection.
Concerns of the public
Right to underwrite
People are becoming aware that they are exposed to global risks, such as rising unemployment, collapse of pension funds, funding problems of welfare programs, and are therefore vulnerable. In this context of cost-shifting, public funding for insurance may be threatened, while community rating in commercial insurance may happen, as for instance with private medical insurance cover in Ireland.
Private insurance is based on mutuality and consequently discriminates in setting premiums. Mutual insurance refers to the notion of forming a risk pool in which each of the members participate according to the risk they represent to the pool. The cost of the insured risk is distributed between the members of the pool, each paying its own part.26 Individuals assessed as representing a higher perceived risk may pay more, and some may be denied cover, although the great majority are treated as standard risks.13,25,27,28
Duty of disclosure
The duty of disclosure, which is established by legislation, states that the insurance applicants must declare everything relevant to their risk’s appreciation and their classification.6 If the applicants have neglected to mention that they are carriers of genetic disorders or that these run in the family, this could be invoked to prove that the applicants have made a false declaration and that the contract is invalid.
The duty of disclosure raises many questions: (1) Are genetic test results always relevant for insurers? Applicants who test positive for genetic mutations in a context of research might not have health problems that are relevant for insurance purposes; (2) How relevant is it, when people neglect to inform their insurer about medical problems or conceal health information from them, if their death has nothing to do with the missing information? (3) Insurers may have access to confidential information that applicants do not want to know, thus infringing on their right not to know. (4) The duty of disclosure may also generate social pressure on a would-be applicant to have a genetic test and disclose a negative result to show that their family history does not put them at increase risk.
Fear of discrimination
The fear of genetic discrimination by insurers or employers may tip the scales against somebody seeking testing to obtain improved medical management and reassurance.29 This fear has been observed among people with a family history of Huntington disease who requested presymptomatic gene identification: people attempted to avoid insurance or employment discrimination by withholding the decision to seek testing from their primary care providers.29 People may also be encouraged not to share the result with their general practitioner for fear of disclosure to insurance companies.30 Genetic testing could then cause insurance applicants and their relatives to be rated up or denied insurance and lead to social exclusion, especially since genetic information would not only be used for insurance purposes but also employment purposes. The practice of some clinicians to advise people to buy insurance before having a predictive DNA-test highlights the current perception that people at high genetic risk of late-onset disease face the additional social disadvantage of higher premiums or application rejection.31
Regulatory frameworks
In regard to the above, two principles govern the use of genetic information and testing in insurance and employment; firstly, no one should be subjected to discrimination based on genetic characteristics; secondly, the disclosure of information to a third party or accessibility to personal genetic data should be allowed only with the individual’s informed consent. These principles can be found in all international and regional texts. There is a general consensus that applicants should not undergo genetic testing as a condition of obtaining insurance.
On the contrary, national texts (legislation and recommendations) vary greatly. Three solutions are usually proposed: (1) Prohibition of any use of genetic information by insurers outright; such as Austria, Belgium, Denmark, Estonia, France, Luxembourg, and Norway. In Belgium, a notable feature of the legislation is that it prohibits the use of genetic information even in circumstances where it is to the benefit of the applicant. The rationale is to protect privacy. (2) Legislation prohibiting this below a certain amount of coverage, like in Sweden, the Netherlands, and the United Kingdom. In the United Kingdom, the government also set up the Genetics and Insurance Committee (1998) whose role is to assess the actuarial validity of genetic tests that insurance companies would like to be able to take into account in setting insurance premiums. And 3) Moratoria; Moratoria are either indefinite (Finland, Germany), or for a limited number of years (France, Switzerland), or still limited to insurance policies which do not surpass a certain value (Sweden, The Netherlands, the United Kingdom).
Among the countries where there is no regulation, bills have been presented, like in Iceland and Switzerland, or states that have ratified the European Convention for the Protection of Human Rights and Dignity of the Human Being with Regard to the Application of Biology and Medicine are bound by it. The Council of Europe’s Convention on Biomedicine upholds that the rights and dignity of humans should be respected with regard to the application of biology and medicine and have primary over the goals of science or society.
CONCLUSION
The Hershey–Chase experiments were a series of experiments conducted in 1952 by Alfred Hershey and Martha Chase that helped to confirm that DNA is genetic material. While DNA had been known to biologists since 1869, many scientists still assumed at the time that proteins carried the information for inheritance because DNA appeared to be an inert molecule, and, since it is located in the nucleus, its role was considered to be phosphorus storage. In their experiments, Hershey and Chase showed that when bacteriophages, which are composed of DNA and protein, infect bacteria, their DNA enters the host bacterial cell, but most of their protein does not. Hershey and Chase and subsequent discoveries all served to prove that DNA is the hereditary material.Overview of experiment and observations Hershey shared the 1969 Nobel Prize in Physiology or Medicine with Max Delbrück and Salvador Luria for their “discoveries concerning the genetic structure of viruses”.
Author: SAMEER AFZAL ANSARI,
GURU GOBIND SINGH INDRAPRASTHA UNIVERSITY , THIRD YEAR , SIXTH SEMESTER , BACHELORS OF ARTS AND BACHELORS OF LEGISLATIVE LAWS